The US pharmaceutical landscape is highly regulated and complex. Manufacturers and distributors face the hit due to ever-changing FDA mandates and DSCSA (Drug Supply Chain Security Act) requirements. In such a situation, any growing pharmaceutical manufacturing enterprise needs an advanced ERP system for pharma company. It offers a robust defense against the legal and financial risks related to non-compliance in the American market.
As a modern tool, an ERP solution for pharma industry can bridge the gap between production and regulations. This blog discusses the key regulations that affect the pharmaceutical industry in the USA. We will also go through some common challenges for pharma company, and get an introduction to SAP Business One for Pharma and Life Science Industry. Let’s start with understanding the compliance risk in the US pharmaceutical sector.
Understanding Compliance Risk in the U.S. Pharmaceutical Industry
Compliance risk looms large in the US pharmaceutical industry. A failure to meet the rigorous standards of the Food and Drug Administration (FDA) causes legal, financial, and reputational damage. The US government introduced the Drug Supply Chain Security Act (DSCSA) in 2025 to maintain full digital accountability with unit-level traceability across the entire supply chain. This act makes any non-compliant product unsellable in the American market.
The impact of non-compliance in pharma products can be severe and goes beyond immediate regulatory fines. Consequences can be dreaded FDA warning letters, product recalls, or consent decrees that can stop production for years. Furthermore, the upcoming transition to the 12-digit National Drug Code with integrated AI-driven analytics has changed the definition of “Current” in cGMP. This definition has included robust data integrity and system validation.
Let’s go through the key regulations that impact the US Pharma businesses.
Key U.S. Regulations Impacting Pharma Businesses
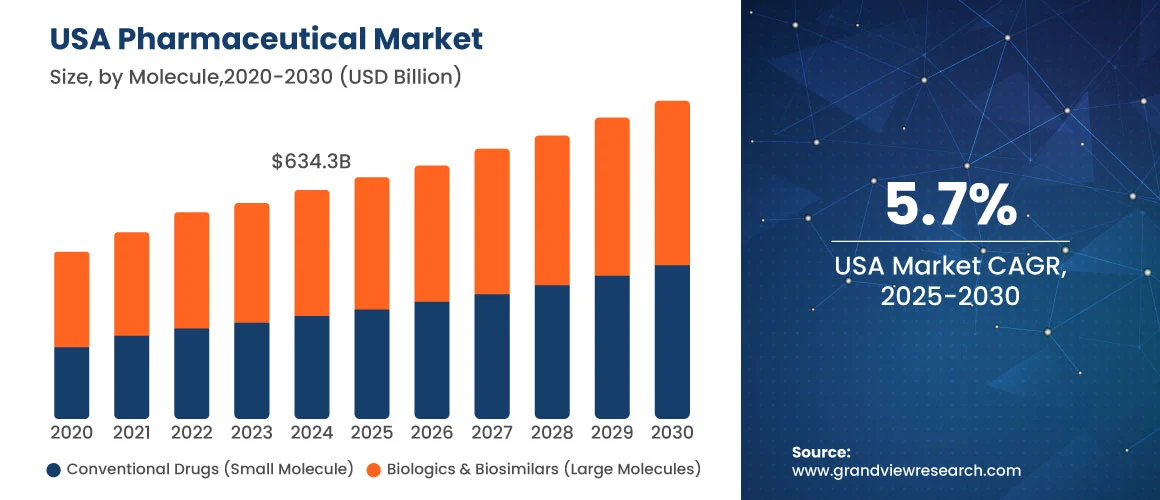
The US Pharmaceutical Market is expected to grow at a CAGR of over 5 percent till 2030. As per the Grand View Research report, this market will reach an estimated value of USD 883.97 billion by the year 2030. Increasing cases of chronic diseases and the geriatric population are key drivers of the steady growth of the pharmaceutical market. However, pharma companies must follow the regulations to leverage the benefits of increasing market size.
The major regulations in the US focus on safety, traceability, and economic transparency. FD, DEA, and CMS primarily govern these regulations. The Drug Supply Chain Security Act (DSCSA) is one of the most important regulations that mandates unit-level electronic traceability. It is useful for preventing counterfeit drugs from entering the market.
Current Good Manufacturing Practice (cGMP) under 21 CFR Parts 210 and 211 is the gold standard for quality and data integrity. The Inflation Reduction Act (IRA) has brought transformative piercing pressures. It forces manufacturers to keep their commercial strategies as per the new federal caps.
DEA regulations govern the distribution of scheduled substances, and the US government has recently updated them to include specific telemedicine prescribing frameworks. The industry prepares for the upcoming transition to NDC-12 (12-digit National Drug Codes).
Common Compliance Challenges for Pharma Companies
Pharmaceutical manufacturers and distributors in the US face several compliance challenges. Mostly, these challenges are based on data integrity and end-to-end supply chain transparency. The most important challenge is to maintain cGMP standards especially when data is in fragmented form. Other challenges include the maintenance of DSCSA through unit-level traceability and logistics hurdles.
A robust ERP system for pharma company can help manufacturers handle auditing and recall management efficiently.
Why Legacy Systems Fail at Compliance
Most legacy systems require manual entry instead of digital accountability. As per the recent standards of the FDA and the DSCSA, data integrity and digitalization are mandatory, and legacy systems fail to maintain them. Fragmented platforms cannot get live shipping information and quality control insights. As a result, pharmaceutical manufacturers cannot achieve the real-time, unit-level serialization, which is mandatory in the US market.
An integrated ERP software like SAP Business One for pharma industry can assist companies to get rid of legacy systems and bring automation in auditing.

How SAP ERP Reduces Compliance Risk for Pharma
As the best ERP with quality management for pharma industry, SAP ERP can mitigate risks related to compliance effectively. Here are its four characteristics that are helpful for the pharmaceutical sector.
End-to-End Traceability & Serialization
SAP B1 enables full compliance with DSCSA mandates by offering unit-level electronic traceability. It covers all the aspects of business from the arrival of raw ingredients to the final selling of products.
Batch, Lot & Expiry Management
SAP Business One automates the monitoring of shelf life and expiration dates. It ensures that any non-compliant or expired materials stay away from production and shipping to customers. In certain cases, SAP can handle targeted, lightning-fast recalls.
Automated Quality Management & Audit Trails
SAP B1 embeds quality checks into the prodcution workflow. It enables manufacturers to adhere to cGMP and 21 CFR Part 11 through timestamped audit trails. It elimiantes the human error found in manual logs.
Regulatory Reporting & Documentation Control
SAP Business One for pharma industry can simplify federal reporting by generating standardized compliance documents. It is useful for maintaining a centralized, version-controlled repository for SOPs and validation records.
Real-Time Data Integrity, Security & Access Control
A robust architecture of the SAP ERP solution prevents data silos to avoid compliance gaps. Effective access controls ensure that only authorized persons can monitor and modify sensitive records. This real-time synchronization keeps compliance data secure and consistent.
SAP Business One for pharma and lifescience industry is an advanced ERP tool for the sector. It offers several business benefits related to compliance and quality control.
Business Benefits of SAP ERP for Pharma Compliance
Implementing SAP Business One for Pharma operations gives a competitive advantage to manufacturers. It transforms compliance into a strategic asset. SAP ERP can automate complex quality checks and serialization workflows to assist the US manufacturers in reducing operational overheads and avoiding delays. As an advanced and feature-rich ERP for pharma industry, SAP Business One keeps every business ready for auditing.
Simply put, SAP Business One is the best ERP with quality management for pharma companies.
Why U.S. Pharma Companies Are Choosing SAP ERP
US pharma companies are increasingly choosing SAP ERP for its ‘compliance-first’ architecture. This ERP solution is capable of meeting the rigours and stringent demands of the FDA as well as the 2025 DSCSA milestones. It eliminates the risks related to siloed data and ensures unit-level serialization with real-time traceability. It also automates complex cGMP workflows to reduce the likelihood of costly Warning Letters or product recalls.
Many manufacturers consider this solution as the best ERP with quality management for pharma sector. This ERP can maintain high data integrity standards to protect brand reputation and patient safety.
How to Choose the Right SAP Partner for Pharma in the USA
The right SAP Partner can focus on technical proficiency and the implementation of a deep regulatory framework of the pharma sector. You need to select the partner with a proven track record of managing FDA compliance and DSCSA traceability requirements. It is also necessary to ensure that the partner can navigate the complexities of 21 CFR Part 11 validation with cGMP methodologies.
A partner with experience in implementing a specialized ERP for pharma industry can help you leverage the benefits of advanced features of SAP B1. As the partner acts as a strategic consultant, you can easily manage the American healthcare supply chain using SAP Business One for pharma.

Conclusion
Stringent regulations and compliance requirements in the USA demand a robust and specialized ERP solution for pharma industry. SAP Business One for pharma can act as a powerful weapon to avoid audit risks and costly recalls. As the American pharma market evolves at a steady pace, it is essential to adopt a modern ERP system for pharma company. Manufacturers can leverage the benefits of an advanced solution by selecting the right partner.
Silver Touch Technologies Inc is a reputable SAP Partner in the USA. Our in-house team of experienced professionals can assist companies in driving growth by handling all the processes from implementation to maintenance. Contact us to learn more about our SAP Business One services and add-ons.
Call to Action
Looking for Giving Your Pharmaceutical Company a Competitive Advantage in the USA? LET’S CONNECT!



